

| Archive Blog Cast Forum RSS Books! Poll Results About Search Fan Art Podcast More Stuff Random |
|
Classic comic reruns every day
|
1 {photo of a spiral fountain}
1 Caption: Pro and Anti
|
First (1) | Previous (3299) | Next (3301) || Latest Rerun (2874) |
Latest New (5380) First 5 | Previous 5 | Next 5 | Latest 5 Annotations theme: First | Previous | Next | Latest || First 5 | Previous 5 | Next 5 | Latest 5 This strip's permanent URL: http://www.irregularwebcomic.net/3300.html
Annotations off: turn on
Annotations on: turn off
|
 Erwin Schrödinger. Public domain image from Wikimedia Commons. |
It was when we discovered that the atoms of the elements were in fact not indivisible that we unlocked the secret of the periodic table. This also explained the mystery of electricity. Atoms were composed of what we describe as positively and negatively charged particles, respectively dubbed protons and electrons. Experiments showed the positive part was large and central, and the negative electrons were relatively tiny and distributed around the atom. Careful measurements determined that there were electrically neutral particles too, called neutrons. These were arranged with the protons in a small nucleus at the core of each atom, the bulk of an atom being empty space buzzing with tiny electrons, like moths around a flame.
This structure also explained the strange phenomenon of radioactivity. Atoms could change into atoms of another element by emitting either an alpha particle (comprised of two neutrons and two protons) or a beta particle (an electron) from their nucleus. In the case of an electron being emitted, the electron was formed by the process of a neutron "breaking apart" into a proton and an electron. In either case, the number of protons in the nucleus changes, and this number is what determines what element the atom is. So elements can be transformed from one into another. We figured out how to force this to happen too. By either splitting atoms or combining atoms we can create new atoms, and potentially release vast amounts of energy. And it turned out that fusing atoms was the power source of the stars, explaining a mystery some people thought could never be solved.
But this is not the end of the story of the atom. The fact that a neutron can fall apart into a proton and an electron hints at something deeper still. The next stage in unravelling the mysteries of the atom occurred rapidly, with a spectacular mixture of theoretical insights and experimental observations.
 Paul Dirac. Public domain image from Wikimedia Commons. |
Just two years later, in 1928, Paul Dirac modified Schrödinger's equation to take into account Einstein's special relativity. He pointed out that the solutions to his new equation allowed the existence of a particle similar to an electron, but with a positive electric charge, opposite to that of the electron. Dirac treated this as a curiosity, and hoped that some future refinement of his theory would resolve the seeming problem. The "problem" was however resolved in a completely different way, when in 1932 Carl David Anderson noticed some strange particle tracks in cloud chamber experiments with cosmic rays. Electrons would produce spiral tracks, influenced by a magnetic field. These new particles also produced spirals, but turning in the opposite direction. This implied they had the same mass as an electron but a positive charge. Anderson had found Dirac's positively charged "anti" electrons. He called them positrons.
Positrons were the first example of antimatter shown to exist - particles that are weird mirror images of ordinary particles. They have the same mass as their normal matter counterparts, but other fundamental properties such as electric charge are the opposite. Another thing about positrons is that if one of them encounters an electron, both the positron and the electron vanish, generating a burst of energy in the form of a pair of gamma rays. All of their mass is converted into energy, leaving behind no particles at all. This reinforces the idea that the positron is a sort of backwards/negative/opposite version of an electron.
In 1930, between Dirac's prediction and Anderson's discovery of the positron, Wolfgang Pauli proposed another theoretical idea to explain a puzzling property of radioactive beta decay. Balancing the energy, momentum, and angular momentum of the particles before and after a beta decay can only be done if there is a third particle involved in the reaction, electrically neutral and extremely light, perhaps even massless. It was later called a neutrino. Untangling this took some time because the existence of the neutron itself was only confirmed by James Chadwick in 1932.
 Irène Joliot-Curie. Public domain image from Wikimedia Commons. |
In a similar way, the light particles, electrons and neutrinos, were later (in 1948) termed leptons (from the Greek leptos, meaning small). Electrons were defined to have lepton number 1. To balance lepton number in the same way as baryon number, the neutrino formed in a beta decay had to have lepton number -1. In other words, it was an anti-neutrino. By building up experimental observations, in a manner very similar to the early chemical experiments of Antoine Lavoisier and his contemporaries, particle physicists realised there were certain rules governing how these particles transformed one into another. Electric charge before and after a reaction was conserved. Baryon number was conserved. Lepton number was conserved.
Further confirmation came from Frédéric and Irène Joliot-Curie (the daughter of Marie and Pierre Curie) in 1934, when they observed a new type of radioactive decay. In their experiments they realised the alchemist's dream of transmuting one element into another, and at the same time discovered something unexpected. They took a sample of aluminium and bombarded it with alpha particles. Some of the particles reacted with aluminium atoms to produce atoms of phosphorus. Aluminium normally has 13 protons and 14 neutrons. Naturally occurring phosphorus atoms all have 15 protons and 16 neutrons (the isotope phosphorus-31). So adding 2 protons and 2 neutrons from the alpha particle should produce exactly what is needed. However, to balance the energy of the reaction, one neutron is ejected, leaving behind 15 protons and just 15 neutrons in the phosphorus atom - a different isotope, phosphorus-30, which is not found in nature. The reason it is never found in nature is that this new isotope is radioactively unstable, and decays within a few minutes. How it decays is interesting.
A nucleus of phosphorus-30 has not enough neutrons to be stable. Looked at another way, it has too many protons. In the resulting decay, a proton turns into a neutron, ejecting a positron and a neutrino. This is sort of the opposite reaction to beta decay, in which a neutron turns into a proton, ejecting an electron and an antineutrino. This new form of decay was called beta-plus decay, or positron emission, and its discovery won the Joliot-Curie's the Nobel Prize for Chemistry in 1935. Positron emission is less likely to occur than beta decay because the total mass of the products can be greater than the mass of the initial particles (depending on the available binding energy of the nucleus), so energy may need to be added in to account for the mass difference. This energy can be available in nuclei that have been transmuted from another nucleus, with residual energy from the first reaction left over and stored as an "excited state" in the nucleus. Finally, for this new form of decay, the neutrino produced is a normal matter neutrino, since its lepton number of 1 is needed to balance the production of a positron with lepton number -1.
 Emilio Segrè. Public domain image from Wikimedia Commons. |
The most characteristic reaction of antimatter particles is annihilation when they meet their normal matter counterparts. We've already mentioned this with electrons and positrons. The same thing happens when an antiproton and a proton meet - they vanish in a burst of energy, released as gamma rays. And as protons are nearly 2000 times as massive as an electron, the amount of energy released is correspondingly higher. And this is where we get our modern science fiction concepts of antimatter engines from. A tiny amount of antimatter can be made to produce an enormous quantity of energy, simply by mixing it with normal matter. Because of the enormous multiplier of the speed of light squared in Einstein's mass-energy equivalence, the amounts of antimatter needed are truly tiny - a few grams can release energy equivalent to all but the largest nuclear bombs ever built. Antimatter annihilation is roughly a thousand times more powerful than any nuclear reaction, because all of the mass is consumed and turned into energy, rather than leaving behind reaction products.
The difficulties in using antimatter as an energy source are manifold. First, you need to create the antimatter. With our current technology, we can create only incredibly small quantities of antimatter, and doing so takes more energy than we can retrieve by using it as a fuel. Secondly, there is the problem of storing it. You can't put antimatter into a fuel tank, because it will react with the fuel tank and destroy it in an enormous explosion. You can't put it in anything physical. This includes air - it will react catastrophically with air just as easily. One potential way to contain antimatter is inside a vacuum chamber with a magnetic field - the theory is understood but building such a device is another thing (not that it's worth doing so, since we don't have enough antimatter to put into it). The third problem is that if anything goes wrong, there's no hope of a harmless fuel leak, or getting a Scottish engineer onto the job and fixing things. Any failure whatsoever means an instant, enormous explosion.
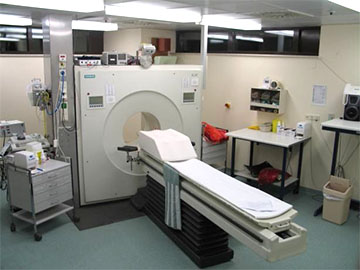 Medical PET scanner. Public domain image by Jens Langner from Wikimedia Commons. |
Antimatter began as a theoretical curiosity, and was later confirmed as a reality. It has been applied to our flights of fancy about the future, as well as to our practical suite of tools for modern medicine. Like many things in science, it was first worked on by cutting edge research proposing odd things that could have no foreseeable practical implications, and has become a valuable part of our modern technology that improves people's health and lifestyles. It's nice to imagine what bizarre things science might possibly do in the future, and then realise that some of those things are a reality right now.
|
LEGO® is a registered trademark of the LEGO Group of companies,
which does not sponsor, authorise, or endorse this site. This material is presented in accordance with the LEGO® Fair Play Guidelines. |