

| Archive Blog Cast Forum RSS Books! Poll Results About Search Fan Art Podcast More Stuff Random |
|
Classic comic reruns every day
|
1 {photo of a blacksmith working red hot iron in a forge}
1 Caption: Blacksmith Physics
|
First (1) | Previous (3222) | Next (3224) || Latest Rerun (2774) |
Latest New (5380) First 5 | Previous 5 | Next 5 | Latest 5 Annotations theme: First | Previous | Next | Latest || First 5 | Previous 5 | Next 5 | Latest 5 This strip's permanent URL: http://www.irregularwebcomic.net/3223.html
Annotations off: turn on
Annotations on: turn off
|
 Not that sort of red hot. |
The hotter an object is, the brighter it glows. The colour changes as well. If you take a piece of iron and heat it in a forge, it first begins to glow a dull red colour. As the temperature of the iron increases, it glows more brightly and the colour shifts through bright red to orange and yellow, until eventually it becomes "white hot".
Blacksmiths and others who work with hot metals know that you can judge the temperature of the metal pretty accurately simply by observing the colour with which it glows. If it's this particular shade of orange, then it's this temperature. In other words, the colour of the glow is determined solely by the temperature, pretty much.
You can use this to determine the temperature of things other than metals too. Our sun is a very bright yellowish-white colour. If you compare it to metals heated to various temperatures, you would guess that the temperature of the sun is about 5800 kelvins. And you'd be right, that is the temperature of the external layer of the sun that is emitting the light, the photosphere. (Other regions of the sun, both inside and outside the photosphere are much, much hotter. The outer corona is transparent though, so we don't see it, and the inner regions are hidden by the photosphere.)
If you extend this with telescopes and sensitive spectrometers, you can also measure the temperatures of stars.
Wait. Stop right there for a second. You can measure the temperatures of stars. Just by collecting their light and comparing the colour to the colours that iron glows when it's heated up. Isn't science wonderful?
 This sort of red hot. Erupting lava on Stromboli, Italy. |
Lord Rayleigh (who we've met before, in the context of Rayleigh scattering and the colour of the sky) and James Jeans tackled this problem in the late 19th century. To do so, they assembled a detailed chain of argument and simplifying approximations, based on their knowledge of physics at the time. The arguments stem from the field of thermodynamics. As the name suggests, this is the study of the movement of heat. And heat, as we know from the work of James Joule in the mid-19th century, is a form of energy embodied by the kinetic energy (energy of motion) of the molecules in an object.
Heat energy can move around in three distinct ways:
 Hot chocolate circulates heat within itself by convection. |
Okay, we have our ideal test object. It absorbs every bit of radiation that hits it, and the only thing it radiates is thermally produced electromagnetic radiation. At low temperature, such an object will look completely black to our eyes. Blacker than any physical object you've ever seen - in fact, you can't see this object, it doesn't reflect any light at all. It would look like a featureless black blot, simply blocking out whatever is behind it. Such an idealised object is called a black body. Whatever radiation comes from a black body is purely thermal radiation, without any contamination from reflected light.
 A real world approximation to a black body. (If this was spherical and frictionless, it would be more ideal.) |
For Rayleigh and Jeans, even this idealised black body object was still a bit too complicated to work with. They considered what sounds conceptually like a more complicated type of black body, but one which made the mathematics and physics easier to think about. Think of an oven or kiln. It's a big empty space at a high temperature. What Rayleigh and Jeans did was to think of this empty space as their black body. Not the oven around it - just the space inside the oven. If you make a small hole in the front of the oven, then you can see the black body. Any incoming radiation that falls on the black body—i.e. into the hole—vanishes into the empty space. In other words, it's completely absorbed - there's absolutely no chance of a reflection[2]. The radiation that emerges from the hole is purely thermal radiation.
Now that we've set up this idealised black body, we can think about what sort of spectrum it has. The spectrum in this context is the distribution of energy along the continuum of wavelengths that make up the electromagnetic spectrum. What wavelengths carry the most energy? What wavelengths carry only a small fraction of it? The approach used by Rayleigh and Jeans was to consider how the waves can bounce around inside the cavity inside oven (i.e. in the "black body").
 Large tiles and small tiles. To span the same distance without cutting, you have a limited selection of large tile sizes, but more choice of small tile sizes. |
Think of it like tiling a room, with the constraint that you're not allowed to cut the tiles. Let's say the room is 10 metres long. If you want to use large tiles, then you are constrained quite severely. You can use 1 metre tiles, and fit exactly 10 of them along the length of the room. If you would rather have 11 tiles, the tiles have to be 10/11 = 0.909 metres long (rounding off). You can't use tiles 0.95 metres long, or 0.97 metres long, or 0.9274 metres long. There's a huge gap in the allowable tile sizes. On the other hand, if you want to use small tiles, you could use 100 mm tiles and fit exactly 100 of them along the room. If you'd rather have 101 tiles, the tiles have to be 100/101 = 99.01 mm long. These tiles are almost the same size! For small tiles, the gap in allowable tile sizes is tiny, so you have a lot more choice of exactly what tile size you'd like to use. Similarly with our black body cavity: At long wavelengths, only a few different, widely spaced wavelengths are allowed. At short wavelengths, there are lots of different wavelengths that fit into the cavity.
 The opening in this empanada oven is a very good approximation of the surface of a black body. The black body is the space inside the oven, not the oven itself. |
The resulting formula that Rayleigh and Jeans first thought of in 1900, and derived more rigorously in 1905, is called the Rayleigh-Jeans law. Here is the formula:
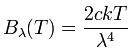
It says that the energy B radiated by a black body at a specific wavelength λ as a function of the temperature T is directly proportional to the temperature, and to 1/λ4. The two constants are c, the speed of light, and k, the Boltzmann constant. I haven't mentioned the Boltzmann constant before - it's the conversion factor between temperature T and kinetic energy (1/2 mv2) of the particles at that temperature (1/2 mv2 = 3/2 kT), so you can probably see why it appears here. Notice that nothing here cares about what the object is made of. All that matters for thermal radiation is how hot the object is.
So now we know why a hot object emits radiation, and we have a theoretical model for exactly how much radiation it should emit and at what wavelengths. This applies to our hypothetical perfect black body. It doesn't work for reflective objects at cool temperatures - say a tennis ball at room temperature. Almost all of the light you see coming off a tennis ball is reflected sunlight (or room light, or whatever), not thermal radiation. But for objects that are very hot—hot enough to glow red-hot or brighter—most of the radiation they emit is thermal and we can mostly ignore the small amount of reflected light. So for many of the things where we are interested in thermal radiation, such as glowing hot iron, or stars, we can treat them as black bodies.
Treating hot objects as black bodies, we can now calculate what their spectrum looks like. A "red hot" object emits most of its thermal radiation around the red end of the visible spectrum. A hotter object emits more in the orange or yellow, and combines to give the object those distinctive colours. An even hotter object emits across the whole visible spectrum, and the combined light appears white to our eyes. Even hotter objects, such as the hottest stars, emit most of their visible thermal radiation in the blue end of the spectrum (but still quite a lot across the rest of the visible range), and the result is the faint bluish colour that some stars have. This colour range, and its strict relation to temperature, is called black-body radiation.
 Lord Rayleigh (left) and James Jeans. Public domain images from Wikimedia Commons. |
This is one of the processes of science. Many of the phenomena around us are incredibly complicated, involving interactions across many different physical processes. To understand, it helps to simplify down to a point where you can make calculations, then go back up the chain to the real world again and understand where your approximations are and when they are important.
 Blacksmiths judge the temperature of hot iron by the colour it glows - using physics! Creative Commons Attribution-NonCommercial image by Flickr user ilker ender. This photo also appears as the title image for this annotation. |
Worse still, if you think of it purely mathematically, the amount of thermal radiation as you go to shorter and shorter wavelengths just keeps getting bigger and bigger, without limit. Every single object in the universe at a temperature higher than absolute zero should be emitting an infinite amount of energy, mostly in ultra-high energy gamma rays! Obviously (a) this isn't happening, so (b) something is wrong with the Rayleigh-Jeans law.
But the Rayleigh-Jeans law is based on our (1900-era) knowledge of physics, and it does work really well for long wavelengths - it gets those almost exactly right. So what's wrong with it?
Albert Michelson wrote in 1903 in his book Light waves and their uses:
The more important fundamental laws and facts of physical science have all been discovered, and these are so firmly established that the possibility of their ever being supplanted in consequence of new discoveries is exceedingly remote.A more famous, but less well verified quotation comes from William Thomson, Lord Kelvin, purported to have said in a speech to the British Association for the Advancement of Science in 1900:
There is nothing new to be discovered in physics now. All that remains is more and more precise measurement.This was the general feeling amongst scientists at the turn of the 19th to the 20th century. As a society we understood motion, atoms, energy, heat, electromagnetism, the nature of light. It was the first time we really felt comfortable in our understanding of everything about the universe. We knew how everything worked.
But there was this one little nagging problem with the spectrum of hot objects...
[2] The incoming radiation may potentially rattle around, reflecting inside the oven and potentially could emerge from the hole again, but we are really considering an idealised oven-like space, in which the walls absorb the radiation and convert it to heat before it has any real chance of emerging from the hole untouched.
[3] It's the specific combination of intensities of these different vibrations at specific higher frequencies (called harmonics) that give a guitar its distinctive sound - that makes it sound different to a piano string, or harp string, or harpsichord string, or violin string, even though all these strings are doing essentially the same thing when sounding the same note.
|
LEGO® is a registered trademark of the LEGO Group of companies,
which does not sponsor, authorise, or endorse this site. This material is presented in accordance with the LEGO® Fair Play Guidelines. |